Steroid Biosynthesis
Genetic Errors
- 17α-Hydroxylase deficiency leads to overproduction of MCs and a deficiency of GCs and sex hormones
- Causes ≈ 5% of cases of adrenal hyperplasia
- SSx: Hypocortisolism causing adrenal hyperplasia, ambiguous genitalia, and hyperaldosteronism
- 21-hydroxylase deficiency leads to low levels of GCs and MCs, and high levels of sex hormones, notably increasing androgen levels in females
- Causes ≈ 95% of cases of adrenal hyperplasia
- SSx: Ambiguous genitalia in females, premature epiphyseal closure, and hirsutism
Physiology of Steroids
- Transcortin (Corticoid-binding globulin) transports GCs and MCs in plasma, SHBG (sex-hormone binding globulin) transport testosterone and estrogen
- Most are metabolized in the liver
- Estrogen is uniquely excreted in the bile, and undergoes enterohepatic recycling, all others are excreted in the urine
- Function by binding to nuclear receptors, dimerizing them, and acting as a transcription factor
Drugs Impacting Steroid Hormone Synthesis
- Aminoglutethimide
- Inhibition of P450scc (CYP11A1, converts cholesterol to pregnenolone)
- Decreases production of all steroid hormones
- Ketoconazole
- Blocks P450scc, 17α-hydroxylase, and 11β-hydroxylase
- Can be used to treat hyperglucocorticoid states
Glucocorticoids
- Regulated by HPT Axis
- CRH released from hypothalamus, causing ACTH (corticotropin) release from anterior pituitary, causing cortisol release from adrenal cortex, which inhibits CRH and ACTH release
- Increase the production of PEP carboxykinase and lipocortin I
- PEP-C increases the rate of gluconeogenesis (raising BG)
- Lipocortin I suppresses Phospholipase A2, inhibiting eicosanoid synthesis
- Activated GC receptor also binds NFκB and prevents its activity as an inflammatory transcription factor
Physiologic Effects
- Liver
- Increased gluconeogenesis and glycogen storage
- Muscle
- Promote breakdown
- Decrease protein synthesis
- Decrease insulin response
- Adipose tissues
- Promote lipolysis
- Decrease insulin response
- Immunosuppression
GC Disorders
Addison’s Disease / Adrenal Insufficiency
- Not the same as Adrenal Fatigue (fictitious Dx used in alternative medicine)
- Primary Adrenal Insufficiency is Addison’s Disease
- Pathophysiology
- Decreased GC release caused by: destruction of adrenal cortex (primary), atrophy due to long-term external GCs (primary), decreased ACTH production (secondary), or decreased CRH production (tertiary)
- Differentiate between primary and tertiary / secondary by evaluating for hypoaldosteronism
- SSx
- Weakness
- N / V
- Anorexia
- Anemia
- Low BP in Addison’s Disease (due to concomitant hypoaldosteronism)
- Hyperpigmentation of skin in Addison’s (ACTH precursor cleaved to increase adrenal output, but cleavage also produces melanocyte stimulating hormone)
- Treatment
- Hydrocortisone 15-25 mg QD or cortisone 20-35 mg QD or Prednisone 3-5mg QD
- Acute Adrenal Crisis: 100mg IV hydrocortisone followed by 200mg QD (NTE 200-400mg QD) continuous infusion x24hr, reduce to 100mg QD once stable, then taper
Cushing’s
- Hypersecretion of adrenal hormones or excessive administration of external GCs
- DDx
- Suspicion of Cushing’s should be investigated with 24hr urine cortisol and low-dose dexamethasone suppression test
- If abnormal, test plasma ACTH and high-dose dexamethasone suppression test
- Low ACTH and no suppression => Adrenal Tumor / Adrenal Cushing’s
- High ACTH and no suppression => Ectopic Cushing’s
- High ACTH and suppression => Pituitary Cushing’s
- Pathophysiology
- Either adrenal tumor, pituitary tumor, or ectopic tumor producing ACTH
- Differentiation by ACTH level (high in secondary and tertiary, low in primary)
- SSx
- Muscle wasting
- “Moon Face”
- Redistribution of fat from limbs to trunk
- Fatty hump
- Osteoporosis
- Immunosuppression / infection
Conn’s Syndrome
-
Lack of 17α-hydroxylase in the adrenal cortex only
-
Leads to GC deficiency and hyperaldosteronism, but adequate sex hormone production
-
Typically caused by an adrenal adenoma
-
SSx
- Hypernatremia
- Polyuria (from hypernatremia)
- Alkalosis
- Alkalosis (via opposite mechanism of Type IV RTA, high export of H+ due to incredible aldosterone levels)
- HTN
Glucocorticoid Properties
| Injectable / PO Steroids | GC Activity | MC Activity | Equivalent Physiologic Dose (mg QD) | Notes |
|---|---|---|---|---|
| Cortisol (Hydrocortisone) | 1 | 1 | 20 | Short Acting |
| Cortisone | 0.8 | 0.8 | 25 | Short Acting |
| Prednisone | 4 | 0.3 | 5 | Intermediate Acting Prodrug |
| Prednisolone | 4 | 0.3 | 5 | Intermediate Acting Biologically Active |
| Methylprednisolone | 5 | 0 | 4 | Intermediate Acting |
| Triamcinolone | 5 | 0 | 4 | Intermediate Acting More hydrophilic Low PO Bioavailability |
| Dexamethasone | 25 | 0 | 0.7 | Long Acting Lipophilic |
| Betamethasone | 25 | 0 | 0.7 | Long Acting Lipophilic |
| Fludrocortisone | 10 | 125 | n/a | Used for MC replacement therapy |
| Topical Steroids | Notes |
|---|---|
| Triamcinolone Acetonide | High Potency |
| Betamethasone valerate | Medium Potency |
| Clobetasol propionate | Very High Potency |
| Halobetasol propionate | Very High Potency |
| Halcinonide | High Potency |
| Fluticasone propionate | Medium potency |
| Mometasone furoate | Medium potency |
Pharmacologic Dosing of Systemic GCs
- All in Prednisone equivalents
- Maintenance: 5-15mg QD
- Moderate: 0.5 mg/kg QD
- High: 1-3 mg/kg QD
- Massive: 15-30 mg/kg QD
- Taper if using ≥ 7.5mg prednisone QD > 3wks by reducing dose by 20mg prednisone equivalents QD
SEs
- Infection
- Muscle weakness / wasting
- Osteoporosis
- Nervousness
- Anxiety
- Insomnia
- Psychosis
- Increased BP and fluid retention
- High BP
- Increased Wt
- Ulcers
- Cataracts
Mineralocorticoids
- Regulated by RAAS
- Increased levels of Ang II leads to increased release of Aldosterone from the adrenal cortex
- Only commonly used drug is fludrocortisone
Androgens
- Testosterone secreted primarily by the Leydig cells of the testes in response to LH
- Testosterone promotes spermatogenesis and Sertoli cells activity
- Testosterone induced feedback inhibition of LH, FSH, and GnRH
- Activin and Inhibin are secreted by Sertoli cells
- Activin stimulates FSH
- Inhibin inhibits FSH
- 5α-reducaste converts testosterone to DHT in target tissues, which is a more potent androgen
- Weak androgens (Androstenedione, DHEA, and DHEAS) are produced in the adrenal glands
Physiologic Effects
- Male secondary sexual characteristics
- Epiphyseal closure
- Increased lean body mass
- Stimulation of erythrocyte production
- Decrease HDL
Synthetic Androgens
- Methyltesosterone (PO)
- Testosterone enanthate (IM)
- Testosterone cypionate (IM)
SEs
- In Women:
- Hirsutism
- Acne
- Amenorrhea
- Clitoral Enlargment
- Deepening of the voice
- In Men:
- Acne
- Sleep Apnea
- Gynecomastia (due to increase in estrogen via mass-balance of testosterone to estrogen reaction)
- Azoospermia
- Hypogonadism
- Psychosis
- Increased agression
Anti-Androgens
- 5α-Reductase Inhibitors
- Finasteride (Proscar, Propecia)
- Used for PCOS, baldness, and BPH
- Dutasteride
- Used for BPH, hirsutism in women, and PCOS
- Finasteride (Proscar, Propecia)
- Steroidal Inhibitors
- Cyproterone
- Used for hirsutism and excessive sexual drive
- Spironolactone
- Used for acne, hirsutism, and PCOS
- Cyproterone
- Non-Steroidal Inhibitors
- Flutamide
- Used for prostate cancer
- Enzalutamide
- Used for metastatic, castration-resistant prostate cancer
- Flutamide
Estrogens and Progestins
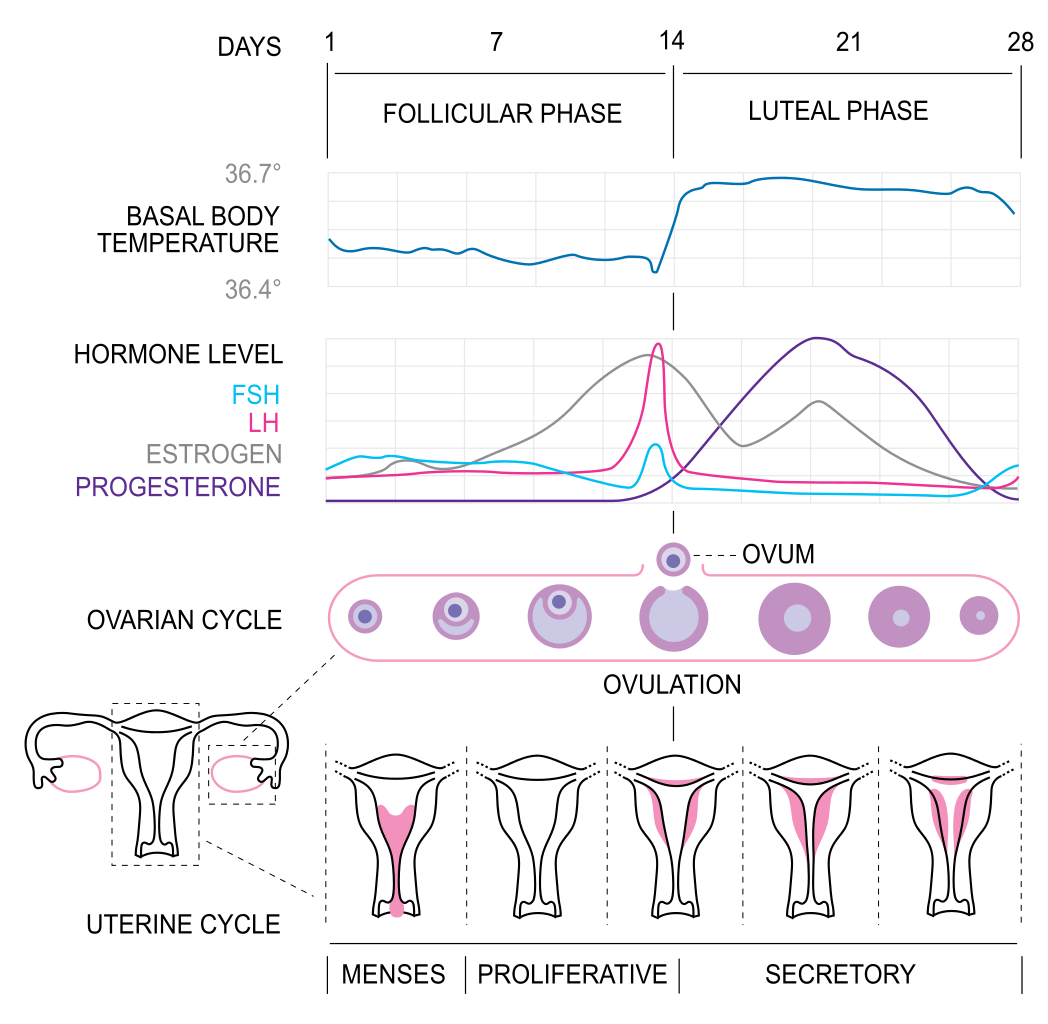
- Estrogen and progesterone are released from the ovaries in response to FSH and LH, and based upon the concentration of estrogen and progesterone, cause either positive or negative feedback regulating the release of GnRH, LH, and FSH
- Estrogen and progesterone suppress FSH in the early follicular phase
- Estrogen and progesterone stimulate the LH and FSH surges leading to ovulation in the late follicular phase
- Estrogen and progesterone suppress LH and FSH in the luteal phase
- Successful implantation leads to the production of hCG by the embryo, which acts in a similar manner to LH, increasing the production of progesterone in the first trimester to maintain the endometrium
- Estrogens are unique among the steroid hormones, as the undergo significant bile excretion and enterohepatic circulation
Physiologic Effects
- Estrogens
- Development and maintenance of breast, ovarian, uterine, and vaginal tissues
- Epiphyseal closure
- Production of female secondary sexual characteristics
- Regulation of CNS including temperature and mood
- Increased HDL
- Decreased LDL
- Enhanced coagulability
- Decreases bone resorption
- Progestins
- Development and maintenance of uterine and breast tissue
- Maintenance of pregnancy
- Increase basal insulin level and insulin responsiveness to glucose
- Promotes glycogen storage
- Competitive inhibition w/ aldosterone
- Depressant and hypnotic effects
SEs
Estrogens
- Uterine bleeding
- Addition of progestin can help prevent this
- Endometrial hyperplasia, especially if given continuously instead of cyclically
- Risk of endometrial carcinoma if pure estrogen therapy is used
- Progestin reduces the risk of endometrial carcinoma
- Breast cancer
- Progesterone does not decrease risk
- Nausea
- VTE
- HA
- Fluid retention (likely due to cross-reactivity at MC receptors) / Wt gain / HTN
Progestins
- Increased appetite
- Fatigue
- Breast regression
- Breakthrough bleeding (esp. in progestin only BC)
- Wt gain, acne, hirsutism (esp. w/ androgenic progesterones)
- Amenorrhea
Non-Steroidal Estrogens (SERMs)
Agonists
- Diethyl stilbestrol
- Used for advanced prostate cancer
- Risk of adenocarcinomas in women if exposed in utero
- Chlorotrianisene
- Used for menopause, prostate cancer, and postpartum breast engorgement
Partial Agonists
- Tamoxifen (Novaldex)
- Used for treatment of breast cancer and prevention in high-risk women
- Weak estrogen in endometrial cells, bone, and hematologic activity (increased clot risk), but anti-estrogen in breasts
- Toremifene (Fareson)
- Similar to tamoxifen
- Used for advanced breast cancer
- Ospemifene (Ospena)
- Similar to toremifene
- Estrogenic effects in vaginal epithelium
- Used for dyspareunia in post-menopausal women
- Raloxefine (Evista)
- Estrogen Activities
- Prevention of osteoporosis
- Decreased LDL
- Increased clot risk
- Anti-Estrogen Activities
- Decreased breast cancer risk
- No endometrial stimulation
- May cause hot flashes
- Estrogen Activities
- Bazedoxifene
- Similar to raloxefine
- Clomiphene (Clomid)
- Increased FSH and LH secretion due to inhibition of estrogen-based inhibition
- Used to stimulate ovulation in women with PCOS, amenorrhea, and ovulation
Antagonists
- Fluvestrant (Faslodex)
- Used to treat breast cancer, particularly in its resistant to tamoxifen
Aromatase Inhibitors
- Used to treat tamoxifen resistant breast cancer
- Drugs
- Anastrazole
- Letrozole
- Exemastane
Progestins
- All given as contraceptives
- Combinations of estrogens and progestins will inhibit LH and FSH release inhibiting ovulation
- Progestin-only do not always inhibit ovulation
- Changes cervical mucus and uterine endometrium to decrease likelihood of fertilization and implantation
- Enlarge breast tissue and suppress lactation (only in combination with estrogens)
- 1st Generation
- Norethindrone
- Ethynlodiol diacetate
- Medroxyprogesterone Acetate
- Depot injection
- 2nd Generation
- Levonorgestrel
- Good PO bioavailability
- Norgestimate
- Prodrug converted into levonorgestrel
- Levonorgestrel
- 3rd Generation
- Desorgestrel
- Prodrug of etonogestrel
- Etonogestrel
- Used in Nexplanon and Nuvaring
- Similar to levonorgestrel
- Desorgestrel
- 4th Generation
- Drospirenone
- Weak activity (10% of levonorgestrel)
- Anti-MC and anti-androgen activity
- Used to control estrogen SEs in combination therapy
- Drospirenone
- SPRM
- Ulipristal Acetate (Ella)
- Emergency contraceptive
- Up to 5d after unprotected sex
- Mifepristone
- Antagonist used as an abortificant in combination w/ misoprostol during the 1st 7wks of pregnancy
- Emergency contraceptive
- Danazol
- Inhibition of LH, FSH, and ovarian function
- Atrophy of the endometrium
- Used for endometriosis
- Ulipristal Acetate (Ella)
| Progestin | Androgen | Anti-Estrogen | |
|---|---|---|---|
| Norethindrone | + | ++ | + |
| Levonorgestrel | +++ | +++ | ++ |
| Norgestimate | +++ | - | +++ |
| Desogestrel | +++ | +/- | +++ |
| Drospirenone | + | Anti-Androgen | - |
| Medroxyprogesterone Acetate | ++ | + | ++ |
